LEAD ACID BATTERIES: The 411 for Forensic Engineers
Batteries are energy storage devices, so they can be a source for fires or electrical arcing or even shocks and thus are part of what a forensic engineer might be looking at. With hybrid and electric cars some very high energy applications are going to become more prevalent.
Warning: batteries contain chemicals of varying degrees of acidity, alkalinity, or toxicity. Use protective garments, gloves and eye protection when investigating them and be prepared to take samples with containers capable of holding such chemicals without reactions.
All Batteries were originally broken into two kinds: Primary Batteries and Secondary Batteries. Primary batteries are those that have non-reversible chemical reactions, ie, they are not chargeable: Alkaline (zinc-manganese dioxide or oxy-nickel hydroxide), Zinc-carbon. Secondary batteries are those that have a reversible chemical reaction: lead acid, Nickel-Cadmium (NiCd, NiCad™), Lithium Ion, Nickel-Metal-Hydride etc.
All of these have their various characteristics that are of interest for use: cost, (milli-) Amp-Hours, Voltage/cell, weight, size, energy density, power density, etc. For purposes of forensics it is more likely you will be interested in how they work and roughly how they are constructed.
This article will take up the lead-acid battery.
Lead-acid batteries are based upon acid (sulfuric) reacting with metal (lead). There is evidence that metal/acid batteries were known around 2000 years ago. The “Baghdad Battery” or “Parthian Battery” can be researched for those having an interest in the history of batteries. One can also make a battery with a nickel, penny and a lemon or potato. I found it to be about .5 volts with a lemon.
Cells and batteries and packs.
Cells are compartments (just like a jail cell is a compartment) wherein electrical potential can be developed electrochemically. Their voltage can range from roughly 1.2 volts to 2.6 volts. Originally cells did not have enough voltage to be useful so they were combined together in parallel: batteries. E.g. a car battery has 6 cells. The word “battery” comes from using cannons in groups called ‘batteries’ (but the word itself means something beaten or forged, thus a cannon is a forged thing). In more modern uses there are single cell batteries: the common flashlight battery is only a single cell.
A battery pack is when batteries or cells are joined in parallel or in series.
Lead-Acid batteries fall functionally into two major categories: SLI (starting, lighting and ignition) and Deep cycle batteries. The difference being, the SLI type batteries have more plates in the cells to provide surface area for great current generation, but are not tolerant of being heavily discharged. The Deep cycle batteries are optimized with fewer and thicker plates and a greater tolerance for being discharged to lower levels.
Lead acid batteries fall into approximately three versions: liquid (traditional), gel, and AGM (Absorbed Glass Matt).
Liquid (or “wet”) are either maintenance free or serviceable. Maintenance free do not have the handy caps you can loosen and check the chemistry on. These tend to be used for SLI applications.
AGM are sometimes call “sealed regulated valve”, “dry cell”, “non-spillable” or “sealed” lead acid batteries. They are considered to be good for deep-cycle applications, but are also preferred where the possibility of a spill exists.
Gel are so called because there are additives to make the sulfuric acid solution ‘stiff’. They are usually best used for very deep cycle application, but must be charged with a lower charging voltage.
Both the Gel and AGM are also called “Valve Regulated Lead Acid” batteries. These batteries are mistakenly called “sealed” batteries, but that would be explosively dangerous when being overcharged. They are, however, ‘recombinant’ batteries because they are designed to have the generated oxygen (at the positive plates) and hydrogen (at the negative plates) recombine into water, rather then escape, except under sufficient pressure. Thus the ‘regulated’ valve.
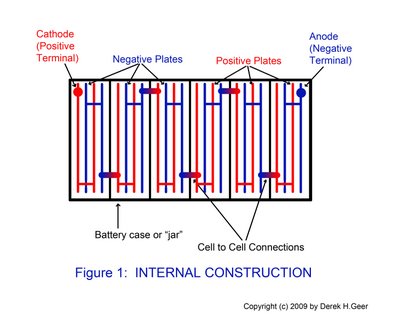
Construction: Lead acid batteries are lead plates in a 33% sulfuric acid solution. The plates are alternately connected together within the cells, and the cells are tied together (electrically) in series. (See Figure 1)
Batteries are energy storage devices, so they can be a source for fires or electrical arcing or even shocks and thus are part of what a forensic engineer might be looking at. With hybrid and electric cars some very high energy applications are going to become more prevalent.
Warning: batteries contain chemicals of varying degrees of acidity, alkalinity, or toxicity. Use protective garments, gloves and eye protection when investigating them and be prepared to take samples with containers capable of holding such chemicals without reactions.
All Batteries were originally broken into two kinds: Primary Batteries and Secondary Batteries. Primary batteries are those that have non-reversible chemical reactions, ie, they are not chargeable: Alkaline (zinc-manganese dioxide or oxy-nickel hydroxide), Zinc-carbon. Secondary batteries are those that have a reversible chemical reaction: lead acid, Nickel-Cadmium (NiCd, NiCad™), Lithium Ion, Nickel-Metal-Hydride etc.
All of these have their various characteristics that are of interest for use: cost, (milli-) Amp-Hours, Voltage/cell, weight, size, energy density, power density, etc. For purposes of forensics it is more likely you will be interested in how they work and roughly how they are constructed.
This article will take up the lead-acid battery.
Lead-acid batteries are based upon acid (sulfuric) reacting with metal (lead). There is evidence that metal/acid batteries were known around 2000 years ago. The “Baghdad Battery” or “Parthian Battery” can be researched for those having an interest in the history of batteries. One can also make a battery with a nickel, penny and a lemon or potato. I found it to be about .5 volts with a lemon.
Cells and batteries and packs.
Cells are compartments (just like a jail cell is a compartment) wherein electrical potential can be developed electrochemically. Their voltage can range from roughly 1.2 volts to 2.6 volts. Originally cells did not have enough voltage to be useful so they were combined together in parallel: batteries. E.g. a car battery has 6 cells. The word “battery” comes from using cannons in groups called ‘batteries’ (but the word itself means something beaten or forged, thus a cannon is a forged thing). In more modern uses there are single cell batteries: the common flashlight battery is only a single cell.
A battery pack is when batteries or cells are joined in parallel or in series.
Lead-Acid batteries fall functionally into two major categories: SLI (starting, lighting and ignition) and Deep cycle batteries. The difference being, the SLI type batteries have more plates in the cells to provide surface area for great current generation, but are not tolerant of being heavily discharged. The Deep cycle batteries are optimized with fewer and thicker plates and a greater tolerance for being discharged to lower levels.
Lead acid batteries fall into approximately three versions: liquid (traditional), gel, and AGM (Absorbed Glass Matt).
Liquid (or “wet”) are either maintenance free or serviceable. Maintenance free do not have the handy caps you can loosen and check the chemistry on. These tend to be used for SLI applications.
AGM are sometimes call “sealed regulated valve”, “dry cell”, “non-spillable” or “sealed” lead acid batteries. They are considered to be good for deep-cycle applications, but are also preferred where the possibility of a spill exists.
Gel are so called because there are additives to make the sulfuric acid solution ‘stiff’. They are usually best used for very deep cycle application, but must be charged with a lower charging voltage.
Both the Gel and AGM are also called “Valve Regulated Lead Acid” batteries. These batteries are mistakenly called “sealed” batteries, but that would be explosively dangerous when being overcharged. They are, however, ‘recombinant’ batteries because they are designed to have the generated oxygen (at the positive plates) and hydrogen (at the negative plates) recombine into water, rather then escape, except under sufficient pressure. Thus the ‘regulated’ valve.
Construction: Lead acid batteries are lead plates in a 33% sulfuric acid solution. The plates are alternately connected together within the cells, and the cells are tied together (electrically) in series. (See Figure 1)
Battery Specifications:
Amp-Hours: The amps times the hours it can deliver it. For a 200 Amp-Hour (AH) battery should deliver 20 Amps for 10 hours. In actual practice the slower it is discharged the more energy one can expect.
CCA: Cold cranking amps: Amperage at 0 degrees F delivered for 30 seconds and the voltage maintains above 7.2 volts.
CA: Cranking amps: amperage measured at 32 degrees F.
MCA: Marine cranking amps: Same as CA.
HCA: Hot cranking amps: amperage measured at 80 degrees F.
RC: Reserve Capacity: The number of minutes a fully charged battery can discharge 25 amps, at 80 degrees F until the battery voltage drops below 10.5 volts (for a 12V battery).
Lead Acid Battery Chargers
The best battery charges have three steps in charging a battery:
1. Bulk charging: about 80% of the is charged at the maximum voltage and current rating for the charger.
2. Absorption charge is charging at 14.4 volts until 98% of the total charge capacity is reached.
3. Float Step with a regulated 13.4 volts at 1 amp or less of current. These will charge the battery to 100% and keep it there without dangerous ‘boiling’.
Note: a charger mis-charging the battery can cause damage to the battery.
The VRLA batteries sometimes require special charge curves.
This should give an Electrical Forensic Engineer enough data to investigate a loss involving a lead-acid battery. There is no attempt here to identify all the ways a battery can fail and cause damage. That is up to the Forensic Engineer in the field to figure out. This will, however, give one the terms and some idea of what he is heading in for.
Labels: Expert witness, Fire Investigation, Forensic Electrical Engineering, Forensic Engineering, Forensic Engineers


0 Comments:
Post a Comment
<< Home